技术交流
1.2水处理不足的后果
Consequences of inadequate water treatment
对于灭菌蒸汽的给水,不完全或不充分的水处理的后果,有时可以在灭菌后立即在各个系统位置以视觉进行识别。因此,定期测试水质尤为重要。
The consequences of incomplete or inade- quate water treatment in the case of feed water for sterilization steam can thus sometimes be visually recognised in various system locations immediately following sterilization. For this reason, it is particularly important to regularly test the water quality.
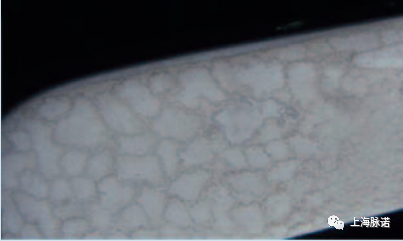
图4:盐残留物 (Salt residues )
2.不锈钢-材料科学的简短课程
Stainless steel – a brief lesson in materials science
除主要的合金元素铁(铁含量通常为>50%)外,非腐蚀(耐腐蚀)铁基不锈钢合金还含有来自添加各种合金元素,如铬、镍、锰,在某些情况下,还有钼等,出于以下目的:
➡以获得所需的性能特性(例如,耐腐蚀性)
➡以确保所需的工作性能
➡提供特定的物理性能,如强度和硬度
In addition to the main alloying element iron (Fe content normally > 50%), non-corroding (rust-resistant) iron-based stainless steel alloys also contain additives from various alloying elements such as chromium, nickel, manganese and, in some cases, molybdenum, etc. for the following purposes:
➡ To obtain the desired performance characteristics (for example, corrosion-resistance)
➡ To ensure the desired working properties
➡ To provide specific physical properties,such as strength and hardness
本领域的技术文献描述了大量可用的铁基不锈钢合金,依据DINEN标准(如10020、10027-1和-2、10028和10088-1、-2和-3)规定的合金成分中的不同合金元素。
The technical literature in this field describes a vast number of available iron-based stainless steel alloys along with the different alloying elements in the alloy composition specified by DIN EN standards such as 10020, 10027-1 and -2, 10028, and 10088-1, -2 and -3.
术语“不锈钢合金”将在下文中使用,一般指所有不受腐蚀的不锈钢,如1.4301、1.4404和其他类似的钢。一般来说,要区分奥氏体、铁素体、马氏体和铁马氏体双相合金。在于他们不同等级的耐腐蚀性能。
The term 'stainless steel alloy' will be used throughout the text below to generally denote all non-corroding stainless steel such as 1.4301, 1.4404 and other similar steels. Generally, a distinction is made between austenitic, ferritic, martensitic and ferriticmartensitic duplex alloys.
The alloys are distinguished, among other things, by their different levels of corrosion resistance.
在医疗和生物医药领域,不同类型的不锈钢合金的应用根据的是特定目的。例如,由于涉及到特殊的硬度要求,大多数外科手术和牙科器械都是由马氏体不锈钢合金制成的。
In the medical and pharmaceutical fields, dif- ferent types of stainless steel alloys are used depending on the specific purpose. For example, because of the special hardness requirements involved, most surgical and dental instruments are made from martensitic stainless steel alloys.
然而,与奥氏体不锈钢合金相比,马氏体合金在硬化过程中获得了更高的硬度值,并且在进行正确的机械工作时,具有一致的锐度。
However, compared to austenitic stainless steel alloys, martensitic alloys attain much higher hardness values following the hardening process and, when subjected to the right mechanical working, a consistent sharpness.
例如,在使用期间受到显著机械压力载荷下的植入物和设备部件优选用奥氏体不锈钢范围内的不同牌号来制造。从生产的角度来看,这些合金的加工成本通常比铁素体或马氏体不锈钢合金更便宜。
Implants and device components that are exposed to significant mechanical pressure loads during use, for example, are preferably manufactured from different variants across the range of available austenitic stainless steels. From a production standpoint, these alloys are usually less expensive to process than ferritic or martensitic stainless steel alloys.
毫无疑问,全面的、同性能的、坚固附着的、无阻碍的不锈钢合金的典型且可能是最重要的性能特征是其耐腐蚀性。这种化学性质可以归因于富含铬氧化物的相应钝化层的存在。
Without a doubt, the typical and probably most important performance characteristic of stainless steel alloys is their corrosion resistance. This chemical property can be attributed to the unimpeded presence of a full-area, homogeneous and firmly adhering passive layer that is rich in chromium oxide.
从化学和热力学的角度来看,钝化层对于腐蚀保护和不锈钢表面更大的化学惰性是负有责任的。
From a chemical and thermodynamic perspective, the passive layer is what is responsible for corrosion-protected and chemically largely inert stainless steel surfaces.
化学元素铬对钝化层的形成至关重要,因为铬(III)氧化物氧化二铬除了铁和氧化铁之外,是这个完整的钝化层的主要组成部分。
The chemical element chromium is critical to the formation of the passive layer, since chromium(III) oxide (Cr2O3) is the main constituent of an intact passive layer aside from Fe and Fe oxide.
当元素铬(作为不锈钢表面的主要统计成分)与氧气结合时,就会形成不锈钢表面的保护性钝化层,例如从空气中,从含有物理溶解氧的水溶液中,或从其他提供氧的钝化溶液中,并在不锈钢表面形成一层始终富含氧化铬的层。尽管这层的厚度只有13纳米(相当于大约5-10个原子层),但这一层能够在金属和周围环境之间形成化学保护(惰性)屏障,同时允许电子而不允许离子通过,从而不断地阻挡组件表面的电位腐蚀电流。
The protective passive layer of a stainless steel surface forms when the element chromium (as a major statistical constituent of the stainless steel surface) combines with oxygen, e.g. from the air, from aqueous solutions with physically dissolved oxygen, or from other oxygen-providing passivation solutions, and creates on the stainless steel surface a layer that is consistently rich in chromium oxide. Despite an extremely low thickness of just 13 nm (equal to roughly 5– 10 atomic layers), this layer is capable of forming a chemically protective (inert) barrier between the metal and the surrounding environment whilst allowing electrons–but not ions—to pass, and thus constantly blocking potential corrosion currents at the component's surface.
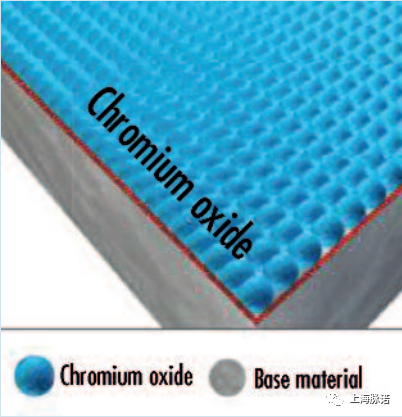
图5:一个完整的钝化层[1]、[2]的模型描述(Model depiction of an intact passive layer [1], [2])
化学上,钝化层主要由氧化铬作为基质组成,包括铁(Fe)、铁氧化物(Fe)、镍(Ni)、镍氧化物(Ni)。对钝化不锈钢表面的俄歇分析和ESCA研究表明,Cr/Fe比>1,而基于根据合金内部成分,Cr/Fe比<0.3。
Chemically, the passive layer is composed mainly of chromium oxide as a matrix with incorporations of iron (Fe) and iron (Fe) oxide and nickel (Ni) and nickel (Ni) oxide. Analytical AUGER and ESCA studies of passive stainless steel surfaces show Cr/Fe ratios > 1, whereas Cr/Fe ratios < 0.3 are usually found in the 'alloy interior' based on the alloy composition.
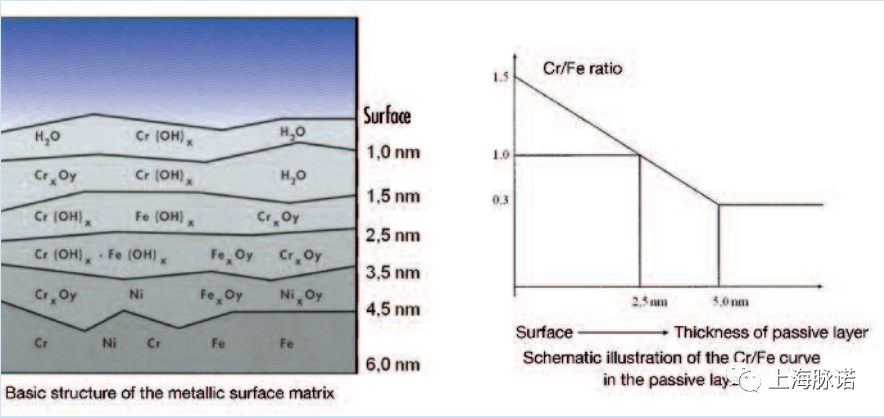
图6:钝化层厚度/铬与铁之比[5](Passive layer thickness / Cr to Fe ratio [5])
不锈钢合金的耐腐蚀材料性能通常是由于其能够形成“富铬-氧化物钝化层”的结果,将组件的表面转换为“钝化”,抗腐蚀或极惰性的状态。在每种独立情况下,非腐蚀性不锈钢合金的化学/热力学钝化属性意味着,即使在主要腐蚀性的环境条件下,也可以避免材料的腐蚀(腐蚀)——每一种不锈钢合金都具有不同的电位耐腐蚀性,特别是“更高等级或更高的合金含量”材料也能抵抗更严重的腐蚀攻击,否则会导致更简单的不锈钢的腐蚀失效,另外——成功的钝化需要不锈钢表面也要经过预处理,以便完全能够钝化。
The corrosion-resistant material behaviour of a stainless steel alloy is generally the result of its capability to form the 'chromium-oxiderich passive layer', which converts the component's surface into a 'passive',non-corroding or extremely inert state.In each individual case, the chemical/thermodynamic passivity of non-corroding stainless steel alloys means that material attack (corrosion) is avoided even under environmental conditions that are predominantly corrosive. To this, however, it must also be added that—each individual stainless steel alloy has a different potential corrosion resistance, and that 'higher grade or higher alloy' materials in particular are also resistant to more severe corrosion attacks that would otherwise cause corrosion failure in simpler stainless steel a lloys,and that—successful passivation requires, among other things, that the stainless steel surface in question also be pre-conditioned such that it is fully capable of being passivated.
|
|
来自:德国的钱伯斯工作组(德语缩写:AKK)的手册


 微信公众号
微信公众号